Water parameters
To keep aquatic animals well, we need to know what fish, crustaceans and other aquatic animals need as water parameters. But also aquatic plants need their own water parameters to do well. I won't discuss nor explain what each kind of aquatic animal or plant needs. That's up to the aquatic keeper to find out. For each fish species need their own parameters. I'm only explaining what the water parameters mean.

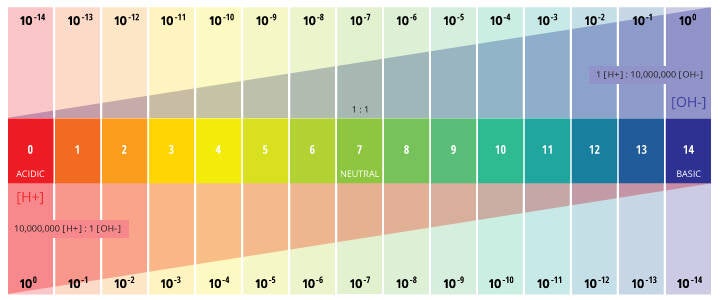
PH: quantitative measure of the acidity or basicity of aqueous or other liquid solutions.
The term, widely used in chemistry, biology and agronomy, translates the values of the concentration of the hydrogen-ion which ordinarily ranges between about 1 and 10−14 gram-equivalents per liter—into numbers between 0 and 14. In pure water, which is neutral (neither acidic nor alkaline), the concentration of the hydrogen ion is 10−7 gram-equivalents per liter, which corresponds to a pH of 7. A solution with a pH less than 7 is considered acidic a solution with a pH greater than 7 is considered basic, or alkaline.
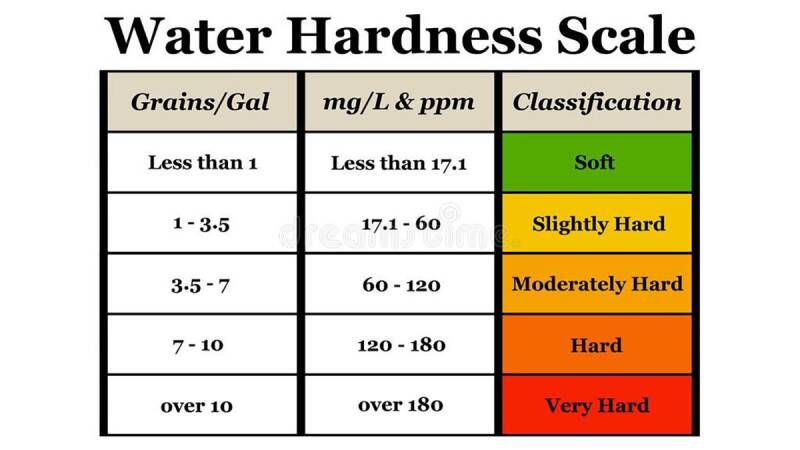
Water hardness: Water hardness is a critical factor in keeping fish and an understanding of this will make it easier to maintain a healthy environment in a fishtank . Water hardness refers to the amount of dissolved minerals in a body of water. In nature water filters through rocks, collecting minerals and other sediments along its journey downstream which determines the hardness of the water for that particular area. Soft water contains less mineral content while harder water contains more. Hardness is measured by Carbonate Hardness(KH) and General Hardness(GH). It is most commonly referred to in German degrees(dKH, dGH, dH), milligrams per litre(mg/L) or parts per million(ppm) of Calcium Carbonate(CaCO3).
KH: Carbonate hardness or Alkalinity refers to the amount of carbonate and bicarbonate ions in the water. Carbonates and bicarbonates are salts of carbonic acid. Carbonic acid is formed when carbon dioxide gas is dissolved in water. The higher the KH, the more stable the pH of the water will be. This is what is referred to as the Buffering capacity of the water, which is the waters ability to maintain and hold a stable pH. Carbonate Hardness can also be referred to as temporary hardness. This is due to the fact that it can be removed through boiling. Carbonate hardness has a direct relationship with the pH of water and when the hardness is high, your pH will also be high and when the hardness is low, the pH will follow and be low.
Note: 1 dKH equals 17.9ppm or 17.9mg/L
GH: General hardness is the measure of dissolved minerals, mainly calcium and magnesium in the water. This is what is commonly referred to as soft or hard water. These minerals hold an electric charge that helps with proper osmoregulation of fish. A higher GH of around 6 – 10dGH is best for a majority of fish and plant life as the ions provide essential electrolytes that do not come from a simple water change. It is better to have a higher GH than a lower GH as this helps to supplement these ions into the aquarium as in nature this happens naturally from the constant flow of fresh water. Using a GH Booster or mineral supplements will help maintain proper amounts of mineral ions that over time are depleted in the aquarium. General Hardness is also referred to as Total hardness or Permanent hardness as it cannot be broken down by boiling.
Note: 1 dGH equals 17.9ppm or 17.9mg/L
To increase the water hardness:
* Sodium Bicarbonate – Baking Soda will raise the KH of the water.
* Crushed Coral is made up mostly of Calcium Carbonate which is ideal for raising water hardness.
* Limestone works also perfectly and will provide calcium and other essential minerals to leech into the water of your fishtank. These are released into the water at a more gradual rate than that of chemical addition.
* And of course, there are commercial resources that can help to increase the KH and GH.
To decrease the water hardness:
To soften hardwater the calcium and magnesium ions need to be removed.
* Rainwater is also used as a way of lowering the hardness of the water.
* Driftwood will soften your aquarium water as it releases tannins gradually over time. Some driftwoods like Mopani are heavy leeching, so this will have a increased effect on your water parameters.
* Peat is another very effective way of softening your aquarium water. It will bind to the calcium and magnesium ions and slowly release tannic acids. Peat can vary in strength and quality and can be unpredictable due to the fact that change can be minimal or extreme. It is better to mix this into a bucket first and then add the required amount to monitor exactly what levels are needed.
* But you can also use reverse osmosis (RO) or a de-ionized (DI) Filter. Both will soften the water. You may want to mix RO or DI water with some normal tap water to get the desired hardness. These filters will also strip out all minerals from the water which are vital for the health of your fish leaving it mineral free. Re-mineralizing the water is essential so the fish can get what they need for proper osmotic function to survive.
Important:
A fishtank with Carbonate Hardness below 3dkH should be monitored more closely as the pH in a fishtank is susceptible to drop unexpectedly due to a low buffing capacity. Over time hardness will be depleted due to the many processes in a fishtank that will produce acids e.g. High feeding rates, a high fish population or long periods between water changes.

NO2: Nitrite. When NO2 is dissolved in water, it produces a 1:1 mixture of nitric acid(HNO3)HNO3) and nitrous acid(HNO2). However, since nitrous acid is unstable in any environment except very cold solution, it decomposes slowly into NONO and HNO3HNO3.
Nitrites have negative impacts on red blood cells and their ability to carry oxygen to the necessary organs.
NO3 :Nitrate. Nitrates occur naturally in our environment in small amounts. It is typically found in rainwater, streams, and in the groundwater table. As nitrogen is found in all living things, it is not unusual to find deposits throughout nature.
The nitrogen cycle is a component of the life-sustaining process and natural nitrate production occurs when bacteria process nitrogen. Nitrate is one of the most soluble forms of nitrogen for plant life.
Safe nitrate levels is lower than 10 mg/L. Ideally at zero.
Removing nitrates from the water:
* There are several methods that can be used to remove nitrates from the water including reverse osmosis, ion exchange filtration, and distillation. Boiling and standard carbon-based filters are not reliable to remove nitrates. Reverse osmosis and ion exchange are the preferred methods for nitrate removal as distillation is neither cost effective nor efficient.
Reverse osmosis is a form of filtration that forces water through membranes that allow only the smallest molecules to pass. These systems are often installed near the kitchen sink and are quite efficient.
Below: An reverse osmosis system.

Ion exchange filtration is more cumbersome and labor intensive. It is effective as the filters absorb nitrate and sulfate ions. Filters must be replaced regularly as any buildup of sulfate can prevent proper ion absorption. These systems are installed near the sink or near where the water enters the home.

Above: Aquatics plants in a tank thrive on nitrates.
Temperature: Temperature is the measure of hotness or coldness expressed in terms of any of several scales, including Fahrenheit and Celsius. Temperature indicates the direction in which heat energy will spontaneously flow—i.e., from a hotter body (one at a higher temperature) to a colder body (one at a lower temperature).


